Avantor Clinical Services supports the need to fast-track clinical trials related to COVID-19 disease progression
Background
The novel SARS-CoV-2 coronavirus is associated with a lower respiratory tract inflammation that often progresses to Acute Respiratory Distress Syndrome ("ARDS") requiring mechanical ventilation.
Clinical Trial Overview
The FDA authorized a phase III double-blind placebo-controlled study for the treatment and prevention of pneumonia associated with COVID-19, which will begin enrolling hospitalized patients with severe COVID-19 infection who are suffering from pneumonia in April 2020. The study will randomize approximately 300 patients.
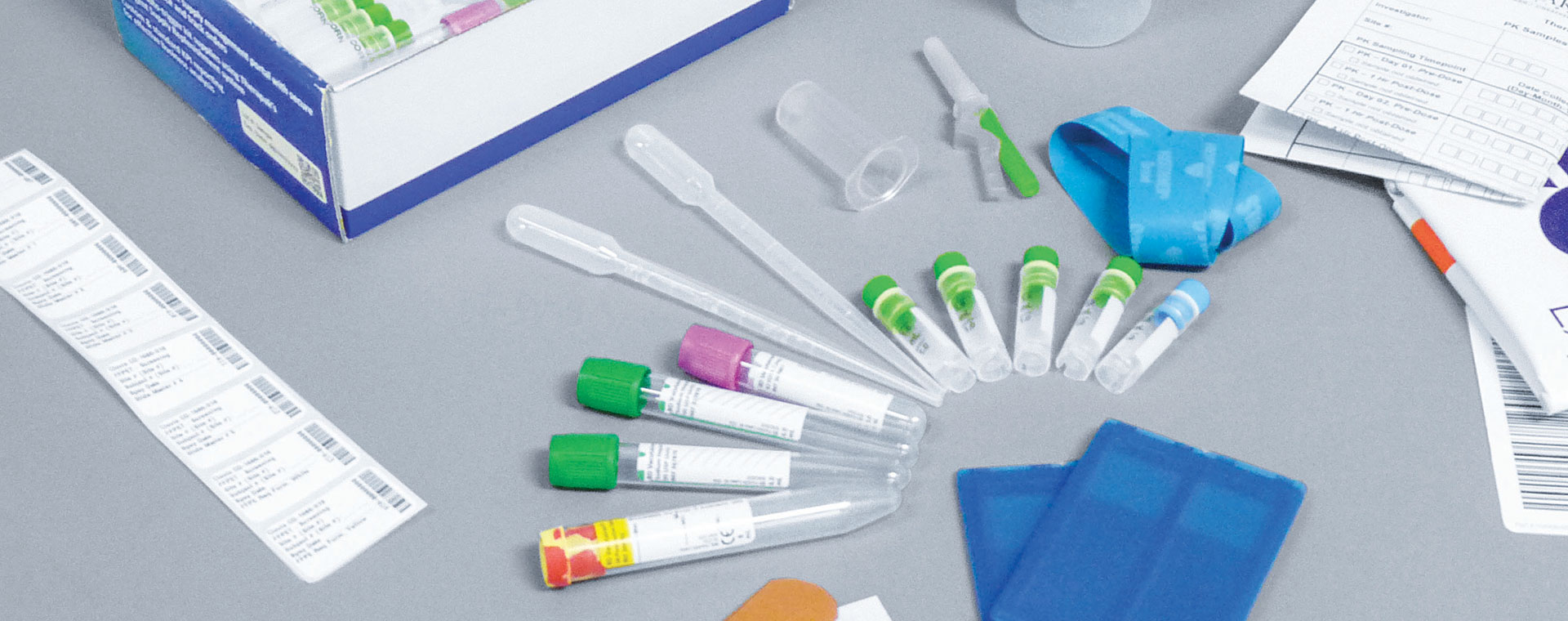
Challenge
Due to the nature of the global pandemic and the need for fast-tracking studies, the customer needed to move quickly in getting their clinical trial underway. They needed a trusted solution to collect and ship their patient samples for their clinical trial. And, they needed an expert resource that could be counted on to not only provide the kitting solutions but also one that could provide the tracking, traceability and logistics the study required – and fast. That’s why they chose Avantor Clinical Services. In less than a week, we provided a custom kitting solution for their clinical trial.
Action/Solution
Understanding the urgency and needs of the customer and the importance of this clinical trial during the COVID-19 pandemic, the Avantor Clinical Services team moved quickly. Through their detailed engagement with the customer and understanding the needs of this high-priority COVID-19 study, the team was able to identify the resources needed and steps required to expedite this request – and begin shipping product in less than one week to the customer.
In order to meet the quick turnaround needs of this fast-track trial:
- The Avantor Clinical Services team assigned a project manager who immediately started the project setup process in parallel with the business development efforts to meet the customer’s expedited timelines.
- Our team worked directly with the customer to understand their protocol supply chain needs, then developed a two-phase approach that consisted of utilizing available bulk supplies as part of the initial phase while we continued developing customized kits that leveraged in-stock and standard items to meet the requirements of the clinical trial.
- The team identified inventory management and logistics support as critical services to ensure proper transport of the biological samples from the various study patients to the selected labs where sample analysis will be performed to determine the safety and efficacy of the study drug.
Results
Through strong collaboration with our customer and among our associates, as well as our deep expertise in clinical trials, we were able to identify the right solution for our customer – and deliver what they needed on time. This enabled them to meet the expedited protocol launch timelines.
We’re proud to be working alongside our customers, enabling their important work.
This is our first COVID-19 Clinical Trial Study project and demonstrates how we are moving science forward to create a better world.



