
Planning & material management
We understand the challenges of managing production environments. From complex processes and quality controls to loss of manufacturing time, we recognize unstable processes risk costly delays, compromising the success of your production activity.
Problem, solution, results
As experts in the life sciences industry, Avantor works side by side with our customers in GMP and critical environments. We’ve been supporting efficiencies in quality procurement and management processes for over 30 years with our range of carefully designed, flexible, innovative services. Whatever the environment and whatever the specification, we help organizations like yours solve complex logistical problems around scientific activities – including working with designated warehouse spaces, managing processes, and conserving cleanroom areas. We maintain ultraclean facilities for scientific research, manufacturing, and other specialized processes – along with many other types of controlled environment.
Accelerating your processes
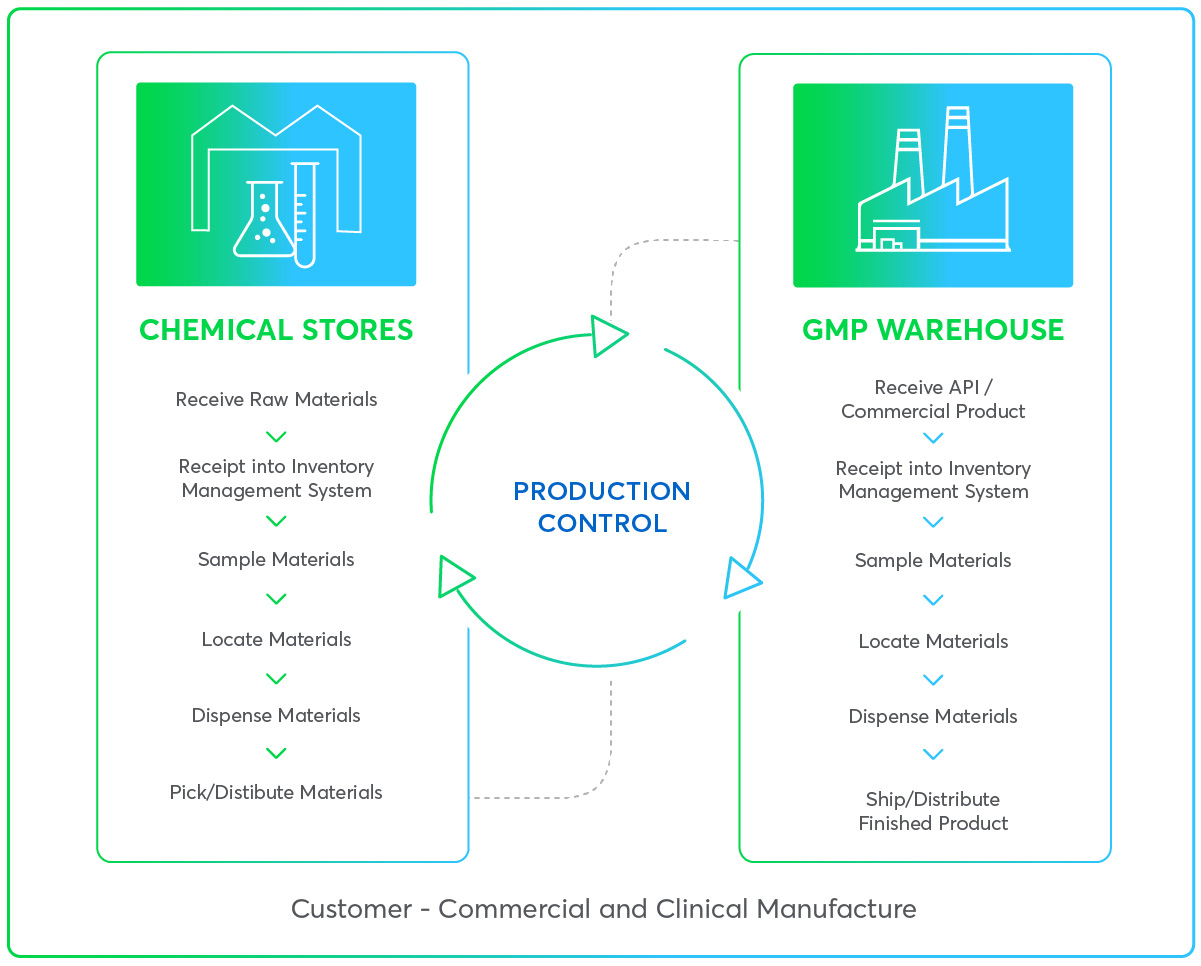
Managing any critical controlled production environment requires expert service. That’s why our trained consultants are available to partner with your teams to support where any deviations require fast, efficient action. Two-way communication ensures complete end-to-end visibility of each project to streamline your operations. From receipt of assets to managing consumables and issuing and shipping materials, we leave you free to focus on your crucial activities. What’s more, the solutions we offer can be fully customized and scalable to meet the needs of your production – no matter the constraints or location.
Through careful assessment of production protocols and processes, we help optimize your approach to reduce or eliminate non-value activities around your workflows - saving valuable time and costly waste. As you would expect, the safe storage of pharmaceuticals is managed carefully and adheres to local mandates (FDA).
Our range of specialization management services include:
- Receipt and QA of raw material sampling
- GMP material sourcing
- Deviation/RCA management
- Change Management (control of warehouse equipment)
- Inventory audits
- GXP training
- GMP & H&S auditing
- Secure GMP document archiving
- EMM (Environmental Monitoring Management)
- Procurement of RM’s & consumables
- Site security for known consignor
Along with the benefits of our expert management and logistics practices, everything is traceable to specific quality GMP standards. The efficiencies of our services pre- and post-API reduce costs and save time – winning back resources for science, leaving you free to concentrate on your core activities.
Avantor GMP capabilities meet regulatory requirements with:
- Compliance: validation of critical instruments, stringent regulatory environments, and custom packing requirements
- Effectiveness: employing the right people and comprehensive training and management (teams are trained to ship dangerous goods by IATA & ADR)
- Quality: secure management systems and support and long-term storage and retention of samples
- Reliability: stock auditing processes ensure levels are never depleted
- Security and protection: with a known consignor, you can be confident your assets are always securely and safely transferred



